
-
Written ByRebecca Kinnet
-
PublishedFebruary 20, 2025
For sterile processing professionals, certification is a key step in demonstrating expertise, ensuring compliance, and advancing in the field. With multiple sterile processing certification options available, understanding the differences between them can help technicians choose the best path for their careers.
Additionally, The Joint Commission introduced the Centralized Sterilization Services (CSS) Certification in January 2024. This certification was created in response to the increasing off-site reprocessing and sterilization trend. Unlike individual technician certifications, CSS certification applies to healthcare facilities and ensures that organizations using centralized sterilization services meet standardized, evidence-based sterilization and disinfection protocols.
In this blog, we’ll break down the main sterile processing certifications available, highlight key differences, and explain what facilities need to know about the new CSS certification.
Key Sterile Processing Certifications
1. Certified Registered Central Service Technician (CRCST)
Offered by the Healthcare Sterile Processing Association (HSPA), the CRCST certification is the most widely recognized credential for sterile processing professionals. It requires:
- 400 hours of hands-on experience
- Passing a comprehensive exam
- Continuing education to maintain certification
2. Certified Sterile Processing and Distribution Technician (CSPDT)
The CSPDT certification, provided by the Certification Board for Sterile Processing and Distribution (CBSPD), is another respected credential. Requirements include:
- At least 12 months of hands-on experience (or equivalent coursework)
- A passing score on the CSPDT exam
- Recertification every five years via continuing education or retesting
3. Certified Instrument Specialist (CIS)
HSPA also offers the CIS certification, which is designed for sterile processing technicians who want to specialize in surgical instruments. To earn this certification, candidates must:
- Hold a CRCST certification
- Gain experience in instrument inspection and testing
- Pass an additional CIS examination
4. Certified Endoscope Reprocessor (CER)
For those handling endoscopes, the CER certification (also from HSPA) focuses on flexible endoscope reprocessing. Requirements include:
- Holding a CRCST or CIS certification
- Passing a specialized exam on endoscope cleaning and sterilization
New Developments: The Joint Commission’s Centralized Sterilization Services (CSS) Certification
Note: This is a facility-based certification not gained by employees or staff.
The Joint Commission has introduced a Centralized Sterilization Services (CSS) Certification to recognize hospitals and healthcare facilities that meet rigorous sterilization and infection prevention standards.
The Joint Commission is providing a new certification to help assure that organizations that provide offsite centralized sterile processing have a standardized, evidence-based approach to high-level disinfection and sterilization for the healthcare organizations which use their services. It is a two-year certification conducted onsite by Joint Commission reviewers.1
In recent years, some healthcare organizations and systems have centralized sterile processing services to an offsite location due to many contributing factors. As medical equipment is moved back and forth from a medical facility to an offsite processing location, maintaining the integrity of the medical instrument, equipment, and devices during transport is critical.
This certification involves:
- On-site evaluation by Joint Commission reviewers
- Assessment of sterilization protocols and quality control measures
- Focus on continuous improvement and patient safety
By earning this certification, healthcare organizations can demonstrate compliance with industry best practices, reduce infection risks, and improve operational efficiency.
State-by-State Certification Requirements
Certification requirements for sterile processing technicians vary by state. While some states mandate certification to work in the field, others allow on-the-job training without formal credentials.
States That Require Certification:
- New Jersey: Requires CRCST or CSPDT certification.
- New York: Mandates certification for all sterile processing technicians.
- Connecticut and Tennessee: Require certification within a set timeframe after employment.
- Delaware
- Pennsylvania
States Without Certification Mandates:
Many states, including Massachusetts, Florida, and Minnesota, do not require certification but highly encourage it and are currently active in legislation or regulation. Most healthcare employers in these states prefer or require certified technicians.
Facility vs. Technician Certification Considerations:
- Sterile processing certification is generally not required on a facility-wide basis. Instead, individual technicians within a facility are often required to obtain certification through a recognized organization like CBSPD or HSPA to demonstrate competency.
- However, in certain states and regulatory situations, facilities may be required to comply with specific sterilization and infection control standards that align with certification requirements.
Ongoing Legislative Changes:
Some states are considering certification requirements to enhance patient safety and standardize sterile processing practices. Technicians must stay informed about evolving regulations.
Below is a current map showing legislative efforts and requirements:
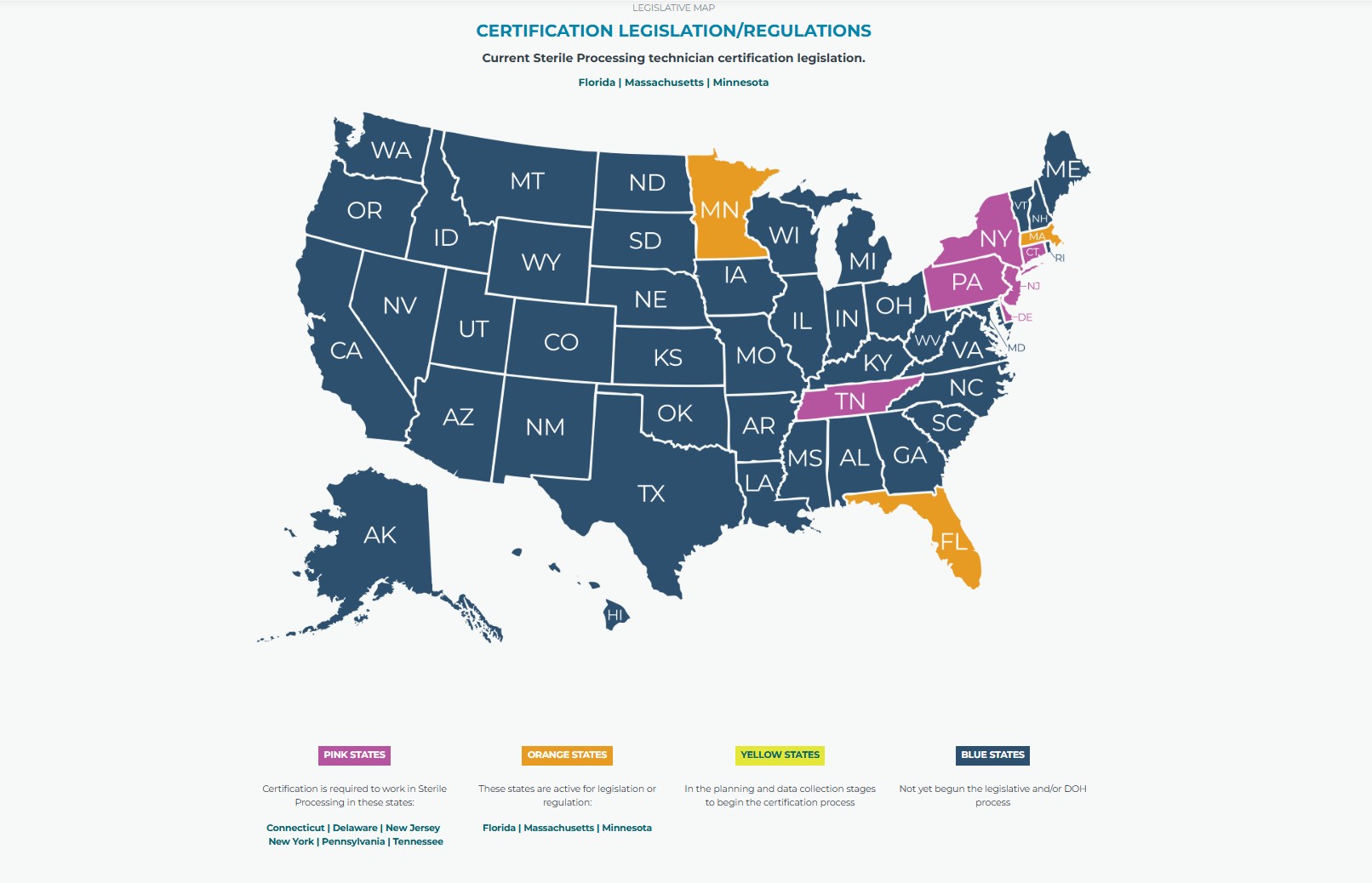
Photo Source: https://myhspa.org/about/advocacy/legislative-certification-issues/
Why Sterile Processing Certification Matters
Even in states where certification isn’t mandatory, obtaining a credential like CRCST or CSPDT can:
- Increase job opportunities and salary potential
- Ensure compliance with industry best practices
- Enhance patient safety and infection control measures
With The Joint Commission’s new CSS certification, healthcare organizations now have an additional pathway to demonstrate excellence in sterile processing and meet the highest standards in patient care.
Skytron: Supporting Sterile Processing Excellence
Sterile processing teams need efficient, high-quality solutions to meet certification standards and ensure patient safety. Skytron offers a full suite of sterile processing solutions, including ultrasonic washers, sterilizers, and workflow-improving technology to help facilities maintain compliance and enhance efficiency.
To learn more about how Skytron supports sterile processing departments, contact us today!
Resources
1. Joint Health Commission
2. HSPA Legislation by State





