
-
Written ByRebecca Kinney
-
PublishedJuly 15, 2024
In August 2023, the Association for the Advancement of Medical Instrumentation (AAMI) released ANSI/AAMI ST108:2023. This new standard outlines the types and quality of water necessary for processing medical devices, setting a new benchmark for safety and efficacy in the operating room and sterile processing.
In August 2023, the Association for the Advancement of Medical Instrumentation (AAMI) released ANSI/AAMI ST108:2023. This new standard outlines the types and quality of water necessary for processing medical devices, setting a new benchmark for safety and efficacy in the operating room and sterile processing. One of our subject matter experts, Tina Cole, helps us understand some of the details in this write-up. Let’s dive into the standard and why it’s so important for patient care.
Understanding the 3 Types of Water in AAMI ST108
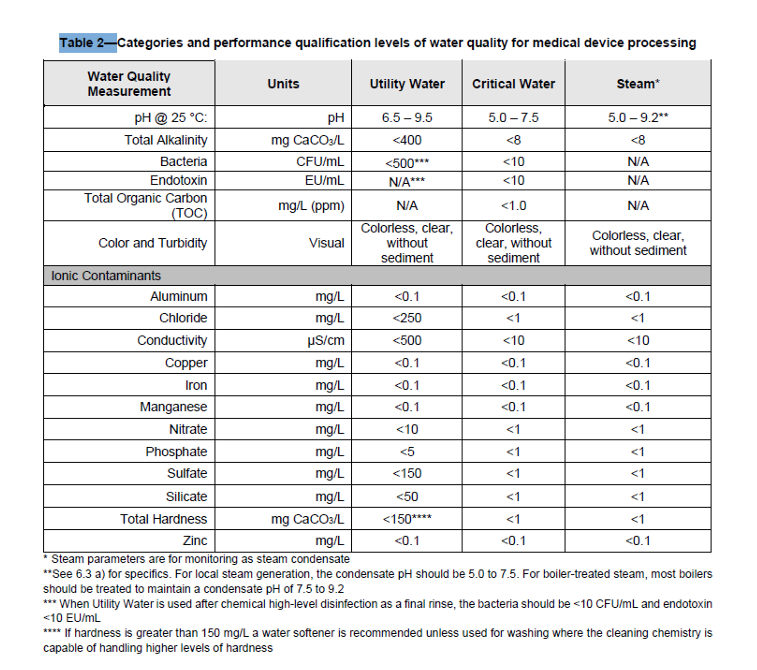
Critical Water: The Gold Standard
Critical Water is the purest form of water outlined in the AAMI ST108 standard. Achieving the stringent quality measurements listed in Table 2 above requires extensive treatment through a multi-step process. This includes:
- Pretreatment
- Primary Treatment (such as Reverse Osmosis [RO] or Deionization [DI])
- Storage and Distribution
- Final Treatment to ensure the removal of microorganisms and organic/inorganic materials
This level of water purity is essential for:
- The final rinse after high-level disinfection
- The final rinse for critical devices before sterilization
- Feedwater for process steam production
Utility Water: The Workhorse
Utility Water starts as tap water and may need further treatment at the facility to meet the quality standards specified in Table 2. Its primary uses are:
- Flushing
- Washing
- Intermediate rinsing (like rinsing between cleaning and disinfection stages)
Steam: The Invisible Essential
Steam, produced by a centralized boiler or a local generator/heat exchanger, also plays a crucial role. When tested as condensate, it must meet the criteria outlined in Table 2 to ensure it’s safe for sterilization processes.
Why Water Quality Matters
Utility Water is the backbone of medical device processing, but for the final rinse, Critical Water is indispensable. Both Utility Water and Critical Water must adhere to the specified requirements at the production point. Critical Water’s bacteria level (<10 CFU/mL) aligns with Clinical & Laboratory Standards Institute (CLSI) guidelines, underscoring its importance in preventing contamination.
Critical Water is especially crucial for instruments contacting the bloodstream or sterile body areas, as it minimizes the risk of introducing endotoxins or microorganisms.
The Processes Behind the Purity
Reverse Osmosis (RO): RO removes most contaminants by pushing water under pressure through a semi-permeable membrane.
Deionized Water (DI): DI water is created by passing source water through ion-exchange beads, which transfer ions from the water to the beads and replace them with hydrogen and hydroxyl ions, resulting in pure water.
Final Rinsing: A Critical Step (AAMI ST108 7.3.1.4)
For devices contacting sterile body areas or the bloodstream, the final rinse must use Critical Water. This water must be free from excessive organic or inorganic contaminants. The quality required for the final rinse depends on the device category:
- Critical and Semi-critical Devices: These require Critical Water to avoid specific contaminants like endotoxins.
- Always follow the device manufacturer’s instructions for use (IFU), which might specify higher water quality standards than AAMI ST108.
Building the Perfect Water Treatment System (AAMI ST108 8.3.2.2)
Critical Water systems are designed to remove dissolved solids and submicron contaminants from feedwater. These systems often include their own pretreatment equipment and are engineered to store, distribute, and further treat water, focusing on eliminating microorganisms and endotoxins. This ensures the water meets the Critical Water specifications in Table 2 and is suitable for final rinsing before sterilization, high-level disinfection, or feedwater for steam generation.
The Bottom Line: Critical Water for Safety
Using Critical Water for the final rinse ensures the removal of residual organic and inorganic contaminants, mitigating many potential issues in subsequent processing stages. Ensuring the highest water quality is vital for the safety and effectiveness of medical devices, directly impacting patient outcomes.
The ANSI/AAMI ST108:2023 standard provides essential guidelines for the water quality used in medical device processing, marking a significant advancement in healthcare standards. By following these guidelines, we can maintain the cleanliness and sterility of instrumentation and devices, ultimately safeguarding patient safety.
Later this month, Skytron will release our all-new processing sink with multiple updates and key features to simplify things for you in sterile processing. To contact our product manager, Peg Brenner, in regards to all stainless products, email pbrenner@skytron.com
Resources
ANSI/AAMI ST108:2023; Water for The Processing Of Medical Devices textbook: Purchase yours here






